…is this gain?
Science Memes
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]
Memes
Miscellaneous
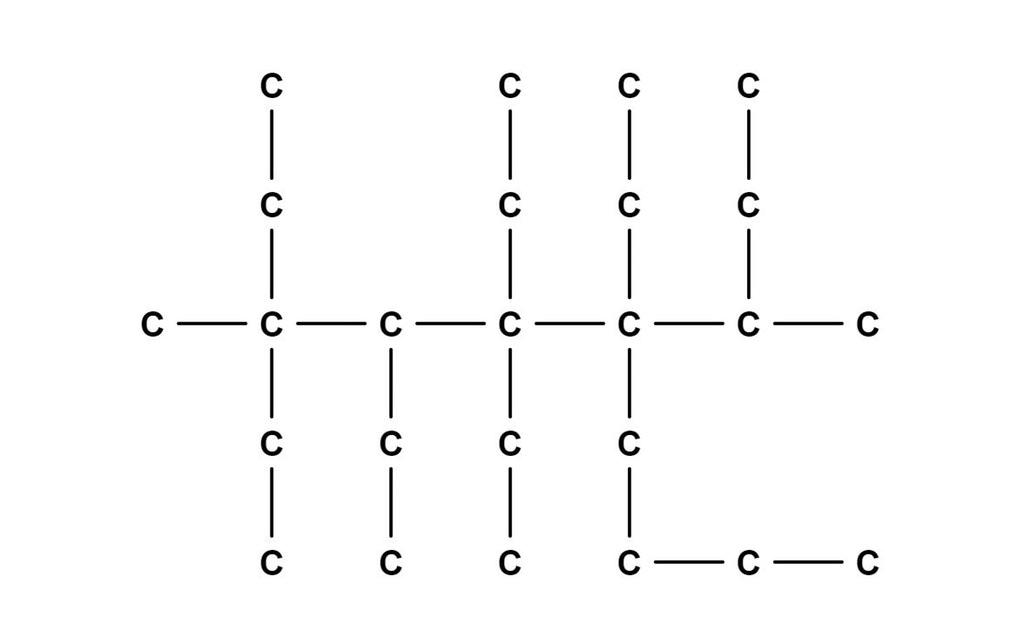
Assuming this has a 47 hydrogens stuck on to make it stable, I'd call it:
3-methyl-3,4,5,5,6-pentaethyl-6-buta-2-yl decane
I am a total chem nitwit, would you like to explain me how you come to this ?
It basically comes down to finding the longest chain of carbons, then you number each of the carbons on that chain and list off things that are attached to that carbon. For example, 1 carbon = methyl, 2 carbons = ethyl, etc.
dodecane has a 12 carbon chain. The longest chain here is 10 carbons, which would be decane.
Good catch.
The urge to add hydrogens to all the carbons not fully bonded is overwhelming
Can someone explain this to me? I don’t get it, but I want to.
Oh by fucking gods. It's loss. People are STILL posting loss. And here I was thinking this was a chemistry meme.
Carbonated loss
Personally I can't wait for this meme to be dead and forgotten
Like that baby
Yeah, me too. I've never even read the webcomic. Can we please just forget about it already?
Loss will live until the last soulless, inspiration destitute and desperately unfunny aging millennial dies a miserable and ugly death.
*Lossane
Lossium.
Couldn’t resist. In a more standard form this molecule should (I believe—chemistry has been a long time ago) look like this:
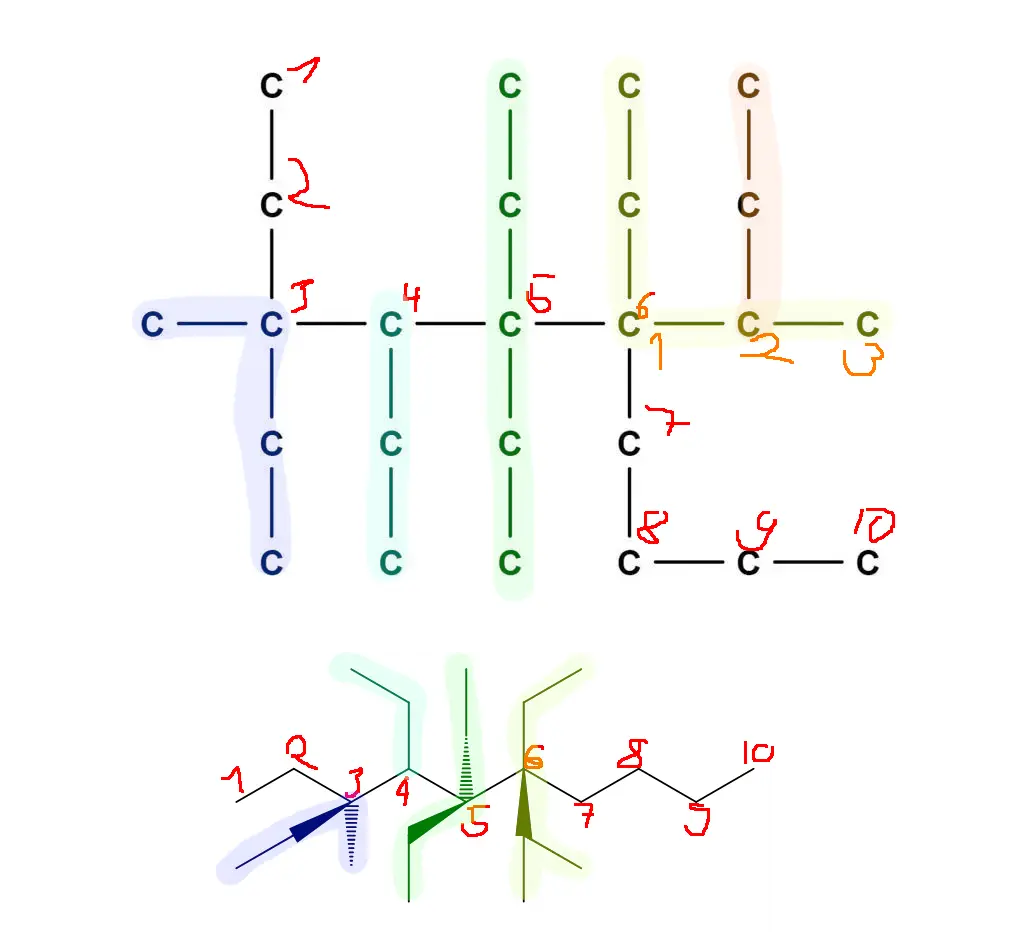
Made with MolView –https://molview.org/?smiles=C%28%5BC%40%5D%28CC%29%28C%29C%28CC%29%5BC%40%5D%28CC%29%28CC%29%5BC%40%40%5D%28C%28C%29C%29%28CC%29CCCC%29C
People knowledgeable in chemistry, please correct!
Change/Invert the steric centers at 3 or 6 and it should be fine.
If I ever teach chemistry to kids imma tell them to name this
My stoned ass thought this was a shifter tree diagram of a goofy little manual transmission for a sec. All gears are just 'crash', lol
3-metilhexa-4,5,5,6,7,7-etil-4-butil From the head, im feel happy now
I'm at a loss
OK how long until it kicks in?
a few years and then you die of cancer
*Cccccccccccccccccccccccccancer
So ... What does this chemical make you lose?
Positive thoughts and other particles with a positive charge.
The game
You lose nothing, don't worry about it.
On the other hand, you learn C (the programming language) by ingesting it, which would be considered a punishment by some.
Sanity
It makes you lose your way in a vast endless c
Your marbles.
I somehow recognized it immediately. I think this meme has rewritten my brain.
Ah ok so it's Loss got it
Fixed the charge on your 3-methyl-3,4,5,5,6-pentaethyl-6-butan-2-yl decane ion, aka lossane.
(It's been a while since I last did chemistry, so apologies if I messed up the nomenclature a bit)
Any idea what a molecule like that would be useful for?
Fuel? Hydrocarbons like that are quite combustible.
actually we start numbering by minimising the number of highest order addition, which is the isobutyl, if it gets same number regardless, then we try to minimise the sum of numbers, so i think it should be called
5-(isobutyl)-5,6,6,7,8-penta-ethyl-8-methyl-decane (I am assuming hydrogen's are present, just not represented, because that usually is the case)
I may alo be wrong here, it has been 4 years since I have been required to do nomenclature myself
Ah, my chemistry department taught me the other way around. I distinctly remember the phrase "methyl-ethyl" being thrown around a bit. Additionaly, we were taught to be more specific about isomers, hence me using butan-2-yl instead of isobutyl.
Then again, scientists often disagree strongly about things like this, so we could both be right. Also, there's a good chance this is just the A-Level specification being weird. I left my old textbook at my student flat, though, so I won't be able to check for a couple of weeks.
I could also be downright wrong myself.
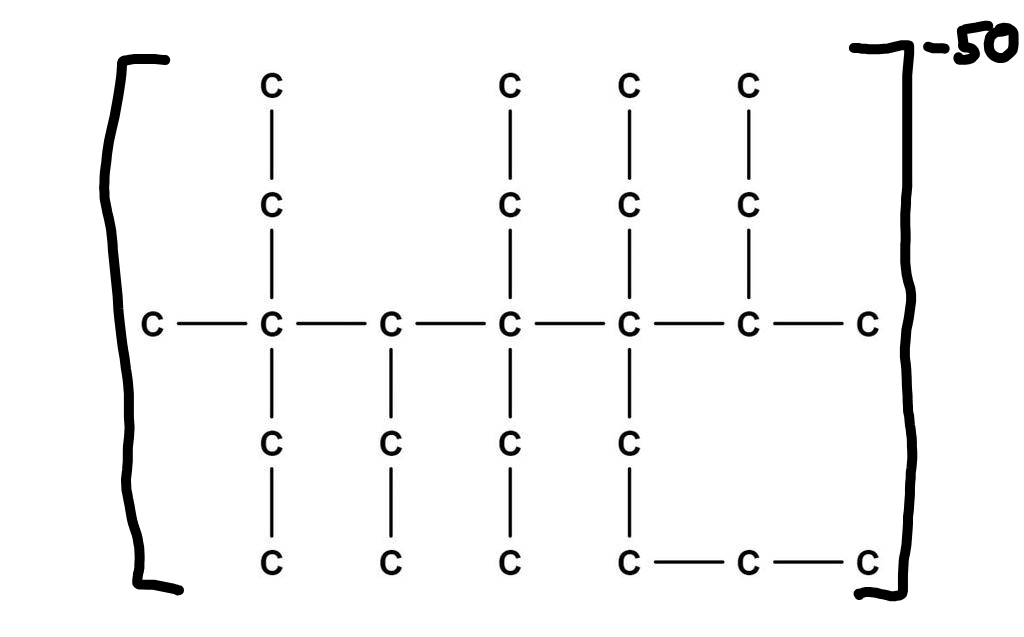
As for the hydrogens, I had assumed they were not present, and that this lossane molecule is an ion with a charge of -50. This is borderline impossible to achieve in real life, of course.
Now i remember we also had to specify what isobutyl (technically just 1 isobutyl exists (not counting stereoisomers), and other form would be tertbutyl). But giving highest priority and minimising sum were definitely something we were taught.
I was free enough to look it up this time - IUPAC guidelines for organic chem - https://iupac.org/wp-content/uploads/2021/06/Organic-Brief-Guide-brochure_v1.1_June2021.pdf (or more generally https://iupac.org/what-we-do/nomenclature/brief-guides/)
Section 7 ( c ) Lowest locant(s) for principal characteristic group(s)
Although I also remember just as we completed our unit on nomenclature, all we got was "common names", now i was supposed to know of the top of my head what a cumene is (which I think is isopropyl benzene (not going to check this one)). Same thing happened with polymers, we were taught IUPAC, and then again, "industrial names"
As someone who paid enough attention in highschool chemistry to get a B, and occasionally watches Nile(red/blue) and E&I videos.... I know some of these words/symbols!
(1,2,2,50)-loss-quinquagintinane