this post was submitted on 28 Apr 2024
820 points (98.2% liked)
Science Memes
10940 readers
2064 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
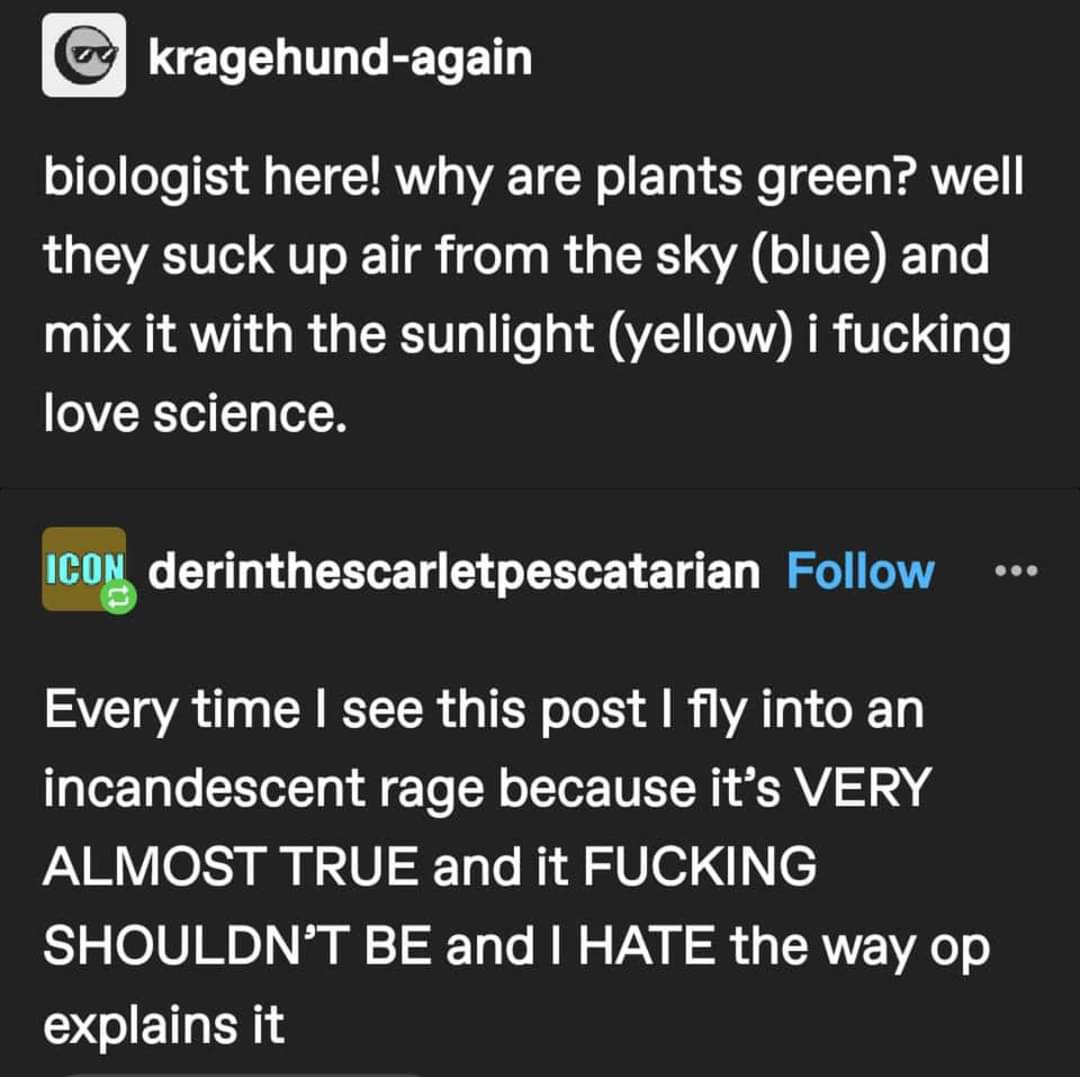
The thing about green photons having too much energy isn't really true, though it's commonly talked about. Blue photons are significantly higher-energy than green, and are very well-absorbed. There's speculation that our sun (being a greenish star) just produces too many green photons, and absorbing so many so fast would be detrimental, but I haven't seen that definitively proven yet. People are trying, though -- there are all sorts of papers about making artificial supplementary antennae to absorb in the green region.
There are a couple proposed reasons to reflect green, which range from information theory arguments about decoupling different parts of the photosynthetic mechanism, to the 'purple earth' hypothesis mentioned in another comment, to the 'green sun' idea. My point is, the why of green photosynthesis is not a settled matter.
Also, the absorbance of red and blue photons isn't because red and blue photons have useful energies, specifically. The photons excite electrons in a 'high energy' path and a 'low energy' path, yes, but the elections excited by these photons don't directly do chemical work -- these exitons are in a quantum-coupled system which is very complicated to understand (I won't even pretend I understand it fully), and the reduction potentials further down the line are only indirectly (and not proportionally) connected to the energies of the original photons.
Basically, we have studied photosynthesis really intensively for like 50+ years, and in some ways it's still basically magic. The more we study it, the more information we have, but more often than not that leaves us more confused, because it's just a crazy system. And I, for one, think that's pretty damn cool.
Will edit later with sources.
You seem like you know a lot more about this topic than I do lol. I've updated my comment to some important context I should have added from the beginning. You can also dig through this thread to see more in depth explanations of lacking context.
The sun is a greenish star and what I was getting at was that a large portion of the Sun's emitted energy is in the form of green light meaning that plants can reduce the magnitude of fluctations in recieved energy by reducing their intake of green light. As you said though, you haven't seen it definitively proven that this is the case.
As for the red a blue wavelengths of light... honestly I don't know nearly enough about photochemistry to talk on the subject and was just parroting what the research paper reported. I am not nearly far enough into quantum to have even heard the term exciton lmao (but I did look them up and they seem cool).
Fantastic to know that plants are just magic actually. Very cool