this post was submitted on 09 Sep 2024
1228 points (92.3% liked)
Science Memes
10356 readers
1925 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
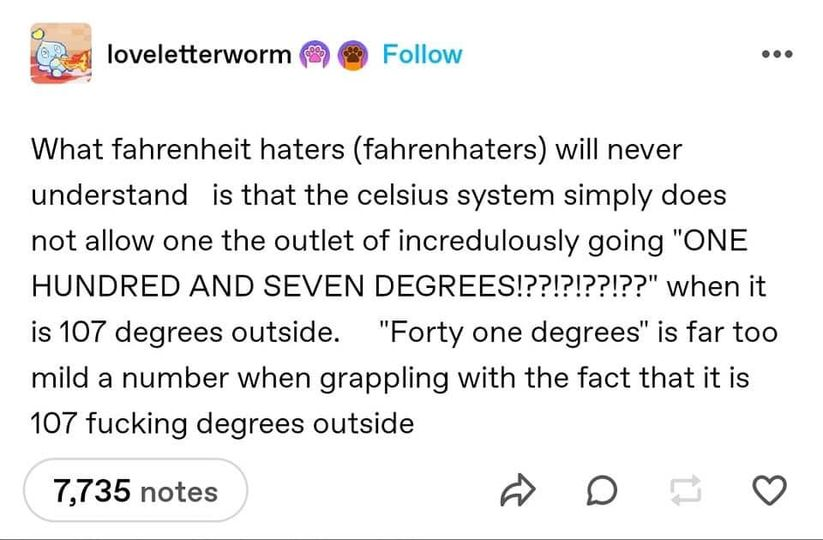
I don't really have a horse in this race but this logic doesn't seem legit to me.
How is -17°C really cold weather AND 37°C really hot weather?
One is actively trying to kill you if weren't already dead by the time the weather got that bad. The other just makes your nuts stick to your thighs -- if you're in a humid place.
I'd agree with the logic if 100F was equal to something like 65°C. 🤷♂️
It makes no sense because that's not what the 0 of the Fahrenheit scale is. The 0 point is the coldest an ammonium chloride brine mixture can be cooled to. The 90 point was an estimated average for human body temperature (it was adjusted up over time). These were chosen because the goal of the scale was to provide a way for people to have a defined temperature scale with a range and degree size that could be reliably reproduced without passing around standardized tools. 100 is really hot because human bodies were used as a reference for the high end, but the low end has nothing to do with the human body.
At what molar concentration? Was it just as much NH4Cl as he could dissolve at ambient temperature and pressure?
As I understand it, yes it was a saturated solution.