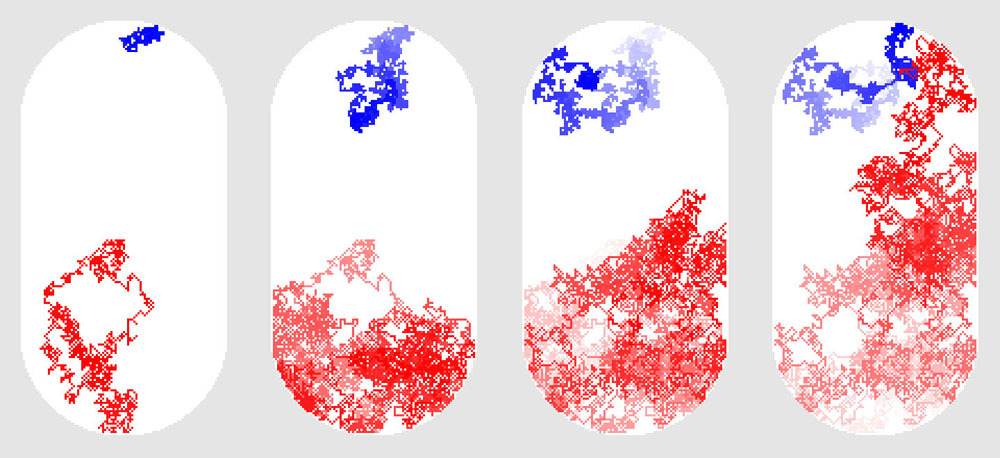
Illustration from The Machinery of Life by David S. Goodsell
Molecules constantly diffuse within the interior of cells, randomly bumping from place to place. This illustration shows several snapshots from a computer simulation of a protein and a sugar molecule diffusing inside a bacterial cell. The path of the protein is shown in blue and the path of sugar is shown in red. They start at opposite ends and explore much of the space inside before they reach each other.
I really like this because it illustrates, more than anything else, that our biomolecules don't "want" to do anything, go some place, bind somewhere; it's just physics and unbelievable quantities of random interactions. Excerpt from the text:
One basic thing remains the same at our size and at molecular size: the solidity of matter. At the scale of molecules, we do not need to worry too much about the odd things that happen with quantum mechanics: to a first approximation, molecules have a definite size and shape, and it is perfectly fine to imagine them bumping into each other and fitting together if the shapes match. If we look closely, their edges may be a bit fuzzy, but for most purposes, we can think of them as physical objects like tables and chairs.
Other properties, however, are very different when we enter the molecular world. For instance, molecules are so small that gravity is completely negligible. The motions and the interactions of biological molecules are completely dominated by the surrounding water molecules. At room temperature, a medium-sized protein travels at a rate of about 5 m/s (the speed of a fast runner). If placed alone in space, this protein would travel its own length in about a nanosecond (a billionth of a second). Inside the cell, however, this protein is battered from all sides by water molecules. It bounces back and forth, always at great speed, but takes a long time to get anywhere. When surrounded by water, this typical protein now requires almost a thousand times longer to move a protein-sized distance.
Imagine a similar situation in our world. You enter an airline terminal and want to reach a ticket window on the far side of the room. The distance is several meters—a distance comparable to your own size. If the room is empty, you dash across in a matter of seconds. But imagine instead that the room is crowded full of other people trying to get to their respective windows. With all the pushing and shoving, it now takes you 15 minutes to cross the room! In this time, you may be pushed all over the room, perhaps even back to your starting point a few times. This is similar (although molecules do not have a goal in mind) to the contorted path molecules take in the cell.
You might ask how anything ever gets done in this chaotic world. It is true that the motion is random, but it is also true that the motion is very fast compared to the motion in our familiar world. Random, diffusive motion is fast enough to perform most of the tasks in the cell. Each molecule simply bumps around until it finds the right place.
To get an idea of how fast this motion is, imagine a typical bacterial cell and place an enzyme at one end and a sugar molecule at the other. They will bump around and wander through the whole cell, encountering many molecules along the way. On average, though, it will only take about a second for those two molecules to bump into each other at least once. This is truly remarkable: this means that any molecule in a typical bacterial cell, during its chaotic journey through the cell, will encounter almost every other molecule in a matter of seconds. So as you are looking at the illustrations in this book, remember that static images give only a single snapshot of this teeming molecular world.